Regulatory compliance
Novozymes ensures that all products are developed, maintained and documented in compliance with current international and national regulatory requirements.

As a responsible company and market leader of industrial enzymes, Novozymes makes sure that all our products can be sold and used legally to all relevant markets worldwide.
Novozymes takes its’ responsibility seriously and has therefore established a large and well-trained global function, monitoring relevant legislation and submitting state-of-the-art dossiers. The Regulatory Affairs function has members in Denmark, France, Canada, the U.S., Japan, Korea, Malaysia, India, China, and Brazil and is interacting with local industry associations and regulatory bodies in order to create transparent legal systems for enzymes.
REACH
Novozymes complies fully with the EU regulations on Registration, Evaluation, Authorization, and Restriction of Chemicals.
The regulations on Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) are issued by the EU. REACH requires all EU-based manufacturers and importers of chemicals to register chemicals that are manufactured or imported in yearly tonnages exceeding a certain level: 1000, 100, 10 and 1 ton(s)/year. The incentive behind REACH is to improve the protection of human health and the environment from the potential risk of chemicals.
Our approach
Novozymes welcomes REACH. In essence, REACH reflects Novozymes’ business strategy and ambition to substitute highly dangerous chemicals with sustainable enzymatic or biological solutions, while ensuring safety of use.
According to the EU legislation, REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals), all EU-based manufacturers and importers of chemicals are required to register chemicals that are manufactured or imported in yearly tonnages exceeding a certain level.
Novozymes’ technical enzymes (used for detergent, leather, special tech, forestry, textile, fuel ethanol etc.) are regarded as chemicals and are therefore subject to the REACH registration. The incentive behind REACH is to improve the protection of human health and the environment from the risks that can be posed by chemicals.
A welcomed step towards sustainable industrial development
Novozymes welcomes REACH as a step towards sustainable industrial development. In essence, it reflects the company’s business strategy to substitute highly dangerous chemicals with sustainable enzymatic or biological solutions, while ensuring safety of use.
Novozymes implements all requirements of REACH as they come into force, in order to ensure uninterrupted supply to customers. Novozymes ensures REACH compliance of products that are manufactured in the EU or imported to the EU/European Economic Area (EEA) by Novozymes A/S in Denmark (if you import Novozymes’ products into the EU/EEA, please inform your Novozymes representative).
As a leading registrant, Novozymes has successfully registered all relevant enzymes and met the REACH deadline 31th May 2018.
Novozymes products are subject to registration for:
Bioenergy
As a manufacturer and importer of enzymes, Novozymes has registered enzymes meeting the REACH registration timeline in 2018.
Novozymes’ solutions for the production of bioethanol contain enzymes, and we ensure REACH registration of these enzymes. Throughout our supply chain, we take care to meet all REACH requirements.
Novozymes has developed exposure scenarios on enzymes specific to the bioenergy industry based on actual measurements using Novozymes products in a bioethanol plant. The exposure scenarios are available for REACH-registered Novozymes products and can be downloaded from Novozymes’ Customer Center. Novozymes also offers safety guidance which can be found in the Library section on Novozymes’ Customer Center.
Household Care
As a manufacturer and importer of enzymes, Novozymes has registered enzymes for household care products meeting the REACH registration timeline 2018.
Novozymes ensures timely REACH registration of our enzymes for the household care industry and throughout our supply chain, we take care to meet all REACH requirements.
In accordance with the International Association for Soaps, Detergents and Maintenance Products’ (A.I.S.E.) descriptions of use and “habits and practices”, Novozymes has developed exposure scenarios. The exposure scenarios are available for REACH-registered Novozymes products and can be downloaded from Novozymes’ Customer Center. Novozymes also offers safety guidance which can be found in the Library section on Novozymes’ Customer Center.
Spray or hard surface cleaning products or medical device cleaning When enzymes are used for spray products or hard surface cleaning or medical device cleaning, exposure of enzymes may exceed the safety level. A.I.S.E. publishes documents on “Exposure measurements of enzymes for risk assessment of spray products” describing how exposure should be assessed for spray products. Novozymes offers instruction about safety for medical device cleaning. If you intend to develop such products, please inform your Novozymes representative for further assistance.
Food and Beverages
Food and beverage products are subject to FIAP compliance, and sometimes also REACH registration is required
Some of Novozymes’ solutions are used both in the manufacturing of food and beverage products and in chemical products. For example, a protein hydrolysate can be used in the food manufacturing industry where the enzyme is subject to FIAP compliance and in cosmetic products where the ingredient is subject to REACH registration. In this case, the enzyme may meet double compliance: FIAP and REACH.
As part of the REACH compliance, Novozymes develops exposure scenarios for our enzymes used in the relevant industries. These are readily available at Novozymes’ Customer Center. Novozymes also offers safety guidance which can be found in the Library at Novozymes’ Customer Center.
Textile - Leather - Pulp and Paper
As a manufacturer and importer of enzymes, Novozymes has registered meeting the REACH registration timeline in 2018.
*Textile, leather and pulp & paper are defined as "articles" under REACH, which may place additional obligations on manufacturers.
Novozymes develops solutions to enhance a range of industrial processes, including textile, leather and pulp & paper production processes. And we ensure timely REACH registration of the enzymes we develop for this purpose. Throughout our supply chain, we take care to meet all REACH requirements.
Textile, leather and pulp & paper are defined as "articles" under REACH. Articles that are manufactured in, or imported to, the EU/EEA trigger obligations for manufacturers/importers if the article contains equal to or more than 0.1% of Substances of Very High Concern (SVHCs.) More information can be found at The European Chemical Agency’s (ECHA) website about Data on Candidate List substances in articles. Novozymes’ solutions for the production of textiles, leather, and pulp and paper are not manufactured with SVHCs or the substances listed on the Candidate List.
The exposure scenarios are available for REACH registered Novozymes products and can be downloaded from the Novozymes Customer Center. Novozymes also offers safety guidance which can be found in the Library at Novozymes’ Customer Center.
Wastewater solutions
As a manufacturer and importer of products for wastewater solutions, Novozymes has registered enzymes meeting the REACH registration timeline in 2018.
Novozymes ensure REACH registration of enzymes as well as REACH compliance of non-enzyme components and throughout our supply chain, we take care to meet all REACH requirements. Some products may contain beneficial living microorganisms, and these are not within the scope of REACH.
The exposure scenarios are available for REACH registered Novozymes products and can be downloaded from Novozymes’ Customer Center. Novozymes also offers safety guidance which can be found in the Library at Novozymes’ Customer Center.
Biopharma
As a manufacturer and importer of products for wastewater solutions, Novozymes has registered enzymes meeting the REACH registration timeline in 2018.
Novozymes ensure REACH registration of enzymes as well as REACH compliance of non-enzyme components and throughout our supply chain, we take care to meet all REACH requirements. Some products may contain beneficial living microorganisms, and these are not within the scope of REACH.
The exposure scenarios are available for REACH registered Novozymes products and can be downloaded from Novozymes’ Customer Center. Novozymes also offers safety guidance which can be found in the Library at Novozymes’ Customer Center.
Do you have a question regarding REACH?
Novozymes’ REACH team works with all aspects of REACH and is dedicated to answering any queries that our customers may have. If you have any questions or need our support with regards to REACH, please contact your Novozymes representative or email the REACH team at reach@novozymes.com.
Novozymes’ products for agricultural solutions are not within the scope of REACH as long as they are used as advised by Novozymes.
Within REACH
Within REACH is Novozymes’ approach to ensuring REACH compliance. The project has been established to ensure that we take all necessary steps to meet REACH compliance and support our customers’ compliance.
Within REACH plays a part in every area of our organization. It is a dynamic structure that communicates with the authorities, suppliers and customers in timely manner. Since REACH is a moving target, Novozymes has established a steering committee to make decisions on all critical issues.
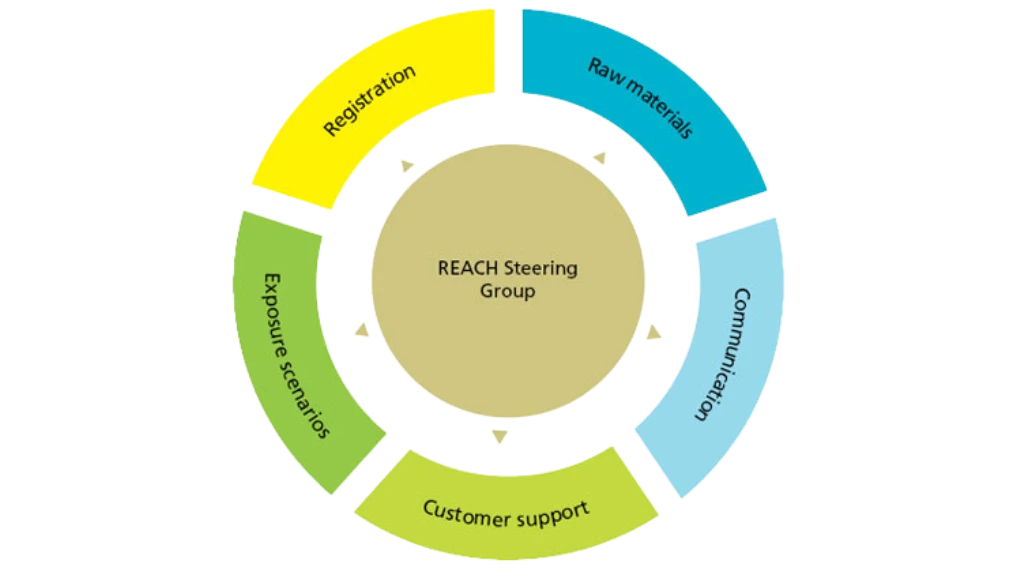
Safety for workers and consumers
With a strong safety policy and more than 40 years of experience in producing enzymes and microbial products, Novozymes offers products that are safe for handling and for use. We also assist and inform our partners about the correct use of our products.
The REACH regulation requires that adequate control of risks is demonstrated for the identified uses of substances. The exposure scenarios that are required by REACH are available for our products (reference: REACH registration number in Section 3 in Safety Data Sheets.) Exposure scenarios for Novozymes solutions can be obtained through Novozymes representatives or Novozymes’ Customer Center.
Raw materials
Novozymes’ exposure to the REACH Candidate and Authorization lists is <1% of our global revenue. We do not use any substance listed in the REACH Authorization List. Boric acid, which has traditionally been used as a common stabilizer for enzymes, is classified as reprotoxic substance as harmonized classification in EU and currently listed on the REACH Candidate List. Over the past years and prior to Candidate List listing of boric acid, Novozymes has not only succeeded in phasing out boric acid from a number of products by developing alternative stabilizers, but also enabled detergent formulators to void the use of boric acid with the introduction of Novozymes alternative stabilizers.
A trustworthy partner for compliance
Manufacturers that use Novozymes’ products in their solutions may have to comply with REACH if their products are manufactured in the EU/EEA or imported to the EU/EEA. As a trusted partner, Novozymes will support our customers’ REACH compliance.
Novozymes works with our supply chain to make sure that relevant raw materials meet REACH compliance.
If you are importing Novozymes’ products into the EU or the EEA, please inform your Novozymes representative.
Novozymes’ enzymes made for the manufacturing of food comply with FIAP in the EU.
FIAP
Novozymes works on complying with FIAP diligently, proactively and with serious commitment in our efforts to be a trustworthy and preferred partner for our customers.
Background
The Food Improvement Agents Package (FIAP) creates a harmonized regulatory system for food enzymes across the European Union.
FIAP applies to all food enzymes sold or used in Europe, including imported enzymes and enzymes used in imported enzyme-treated food.
The regulations have been in force since January 20, 2009 and reached a key milestone on March 11, 2015, the first deadline for food enzyme manufacturers to submit dossiers on all food enzymes currently sold or used in Europe. These submissions are required to ensure compliance of the enzyme industry and its customers when FIAP gets fully implemented in 2023.
Food producers depend on enzyme manufacturers like Novozymes for a reliable supply that is fully compliant with regulatory systems globally. That is why Novozymes has been working on complying with FIAP, and Novozymes is proud to have submitted dossiers for FIAP - covering our existing food enzyme solutions as well as newly launched enzyme products - ahead of the deadline.
Next steps
The safety aspects of the dossiers are being assessed by the European Food Safety Authority (EFSA). The technological need and ensuring that a food enzyme does not mislead consumers is being assessed by the European Commission and Member States.
Based on the results of the assessments, the European Commission will draw up a list of food enzymes to be authorized. This list will become the first EU positive list of approved enzymes. The list is expected around 2023. At this point, FIAP is fully implemented, and only authorized food enzymes that are on the positive list will be allowed to be commercialized and/or used in the production of food sold in the EU. Until then, national provisions apply.
Our approach
To ensure uninterrupted supply of food enzymes to our customers, Novozymes maintains full compliance with FIAP requirements as they come into force.
Novozymes has closely followed the development of FIAP. We remain in close dialogue with the European authorities through the European Association of Manufacturers and Formulators of Enzyme Products (AMFEP) to ensure suitable data requirements for the industry, and to ensure a fast and efficient approval process.
Novozymes has continually submitted dossiers, and well ahead of the deadlines, we submitted FIAP dossiers covering our existing food enzyme solutions as well as newly launched enzyme products. It’s all part of our effort to be a trustworthy and preferred partner for our customers.
Do you have a question regarding FIAP?
Novozymes’ FIAP team works intensively with all aspects of FIAP. If you have any questions or need our support in regard to the implementation of FIAP, please contact the FIAP compliance team at fiap-eu@novozymes.com.
Product information
Novozymes supports proportionate and operational regulations on information to the users of our products, notably on safe handling.
Product labels are the primary communication tool to Novozymes' customers for the safe and effective handling and storage of our products. It is essential that labels convey a clear message on health and safety aspects of our products to ensure that they are handled properly by workers.
Novozymes also implements the Globally Harmonized System (GHS) to ensure the same high level of safety information in all countries where we operate – even where it is not mandated by law. The GHS guidelines were developed by the United Nations to help the entire world communicate in the same way about chemical safety.
Novozymes supports proportionate and operational regulations, as well as the final consumers’ right to know, about the way our products are manufactured. Notably, it has always been a basic principle at Novozymes to openly inform all interested parties about the use of genetically modified microorganisms (GMMs) in our production processes.
To this effect, Novozymes’ policy is to inform our customers, in compliance and sometimes beyond legal requirements. Such information may be part of labels, safety data sheets (SDS), product data sheets, and web-based information.
The information we provide to our customers allows them to comply with relevant legislations. It is also reflected when appropriate, in our customers’ communication with final users and consumers.
Our information policy is implemented and monitored through our Quality Management System. We had no incidents of non-compliance with regulatory labelling requirements in 2016.
Novozymes maintains an ongoing dialogue with stakeholders including legislators and authorities, food producers and retailers, the public and the non-governmental organizations on all above topics.
Toxic substance reduction plan summary
Here you can find a summary of the 2020 public plan on toxic substance reduction.
By law, Novozymes is required to post online the summary of the Toxic Substance Reduction Plan, so as to make it available to the public.
Download the 2020 Public Plan Summary Letter from Novozymes Canada Limited.